Fake Postinor-2 spreads over-the-counter, NAFDAC warns as SFH disowns product.
Favour Rotimi
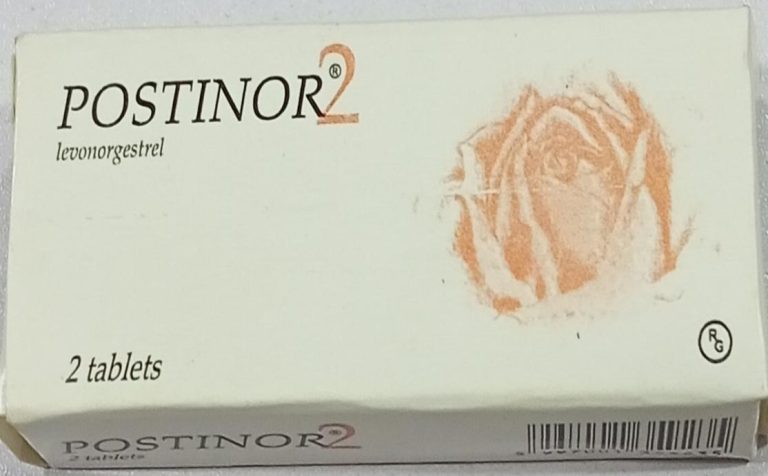
Two falsified versions of the emergency contraceptive pill, Postinor-2 (Levonorgestrel 0.75mg), have been confirmed to be circulating in the Nigeria market.
The National Agency for Food and Drug Administration and Control (NAFDAC) has warned.
The regulatory agency in a recent post on X warns Nigerians against the use of falsified batches of the emergency contraceptive pill in circulation.
The agency disclosed on Monday that the counterfeit versions were detected following a report from the Society for Family Health (SFH), the marketing authorisation holder, which confirmed it did not import the suspect products
NAFDAC explained that the fake contraceptives carry noticeable spelling and labelling errors that distinguish them from the original.
According to the agency, “The font size of the text on the pin verification sticker of the fake appears smaller, with the word ‘Verify’ wrongly spelt as ‘Veify’. On the back of the pack, the fake bears ‘Distnibuted in Nigeria’ instead of the correct ‘Distributed in Nigeria.’”
The genuine Postinor-2, the agency stated, is batch T32458H, manufactured in February 2023, with an expiry date of February 2027 and bears registration number 04-6985.

However, two falsified versions have been confirmed — Counterfeit Type 1, batch T36184B, manufactured in August 2024 with an expiry date of August 2028, and Counterfeit Type 2, batch 332, manufactured in March 2023 with an expiry date of February 2027.
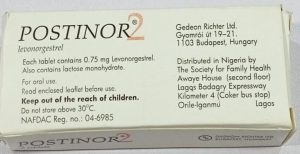
Both fake products also carry the same registration number as the authentic brand, making them potentially misleading to consumers.
NAFDAC warned that the use of the falsified contraceptives could lead to failed contraception, exposure to harmful or toxic substances, unpredictable side effects, and long-term reproductive health complications
In extreme cases, the agency said contaminated ingredients could trigger allergic reactions, organ damage, or even death.
“Counterfeit medicines are unregulated, untested, and illegal, making their safety and efficacy impossible to guarantee. Patients should only obtain Postinor-2 from verified pharmacies or licensed healthcare providers,” the agency cautioned.
It added that investigations into the source of the falsified products were ongoing, while all zonal directors and state coordinators have been directed to intensify surveillance and mop up the counterfeit batches across states.
The agency urged consumers and healthcare workers to carefully verify PIN stickers, report suspicious products, and purchase medicines only from reputable outlets.
In a related post on X, the Society for Family Health (SFH) has launched a campaign to raise awareness against the fake contraceptive pill.
The awareness campaign itemise important things to note before purchasing the contraceptive products and how to Identify the fake product at a glance.
The post partly reads: “In line with NAFDAC’s recent announcement, we are raising awareness about the circulation of counterfeit Postinor 2 in the market.”
“Be assured that the Genuine Postinor 2 product is readily available.
Always purchase Postinor-2 from licensed pharmacies and trusted outlets.
Check packaging details carefully before use.
How to Identify:
-Product Name: Postinor 2
-Batch No: T32458H
-Mfg. Date: 02/2023
-Exp. Date: 02/2027
-NAFDAC REGISTRATION NUMBER (NRN): 04-6985.”
Report any suspected counterfeit products to NAFDAC immediately.” The agency advised.






